Abstract
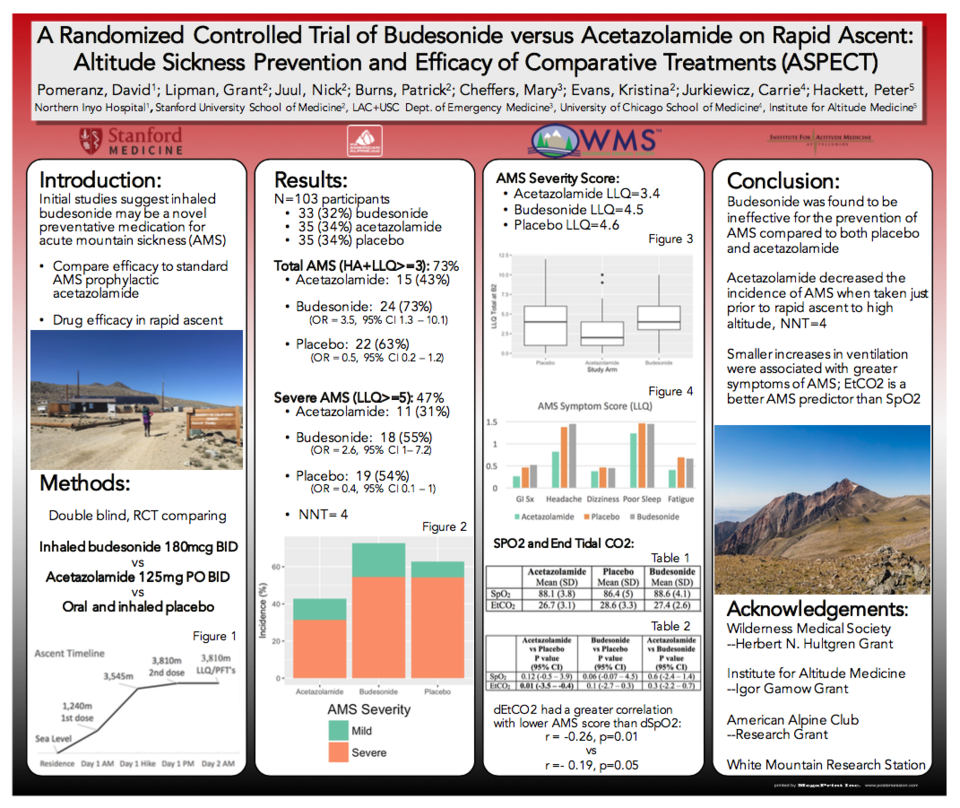
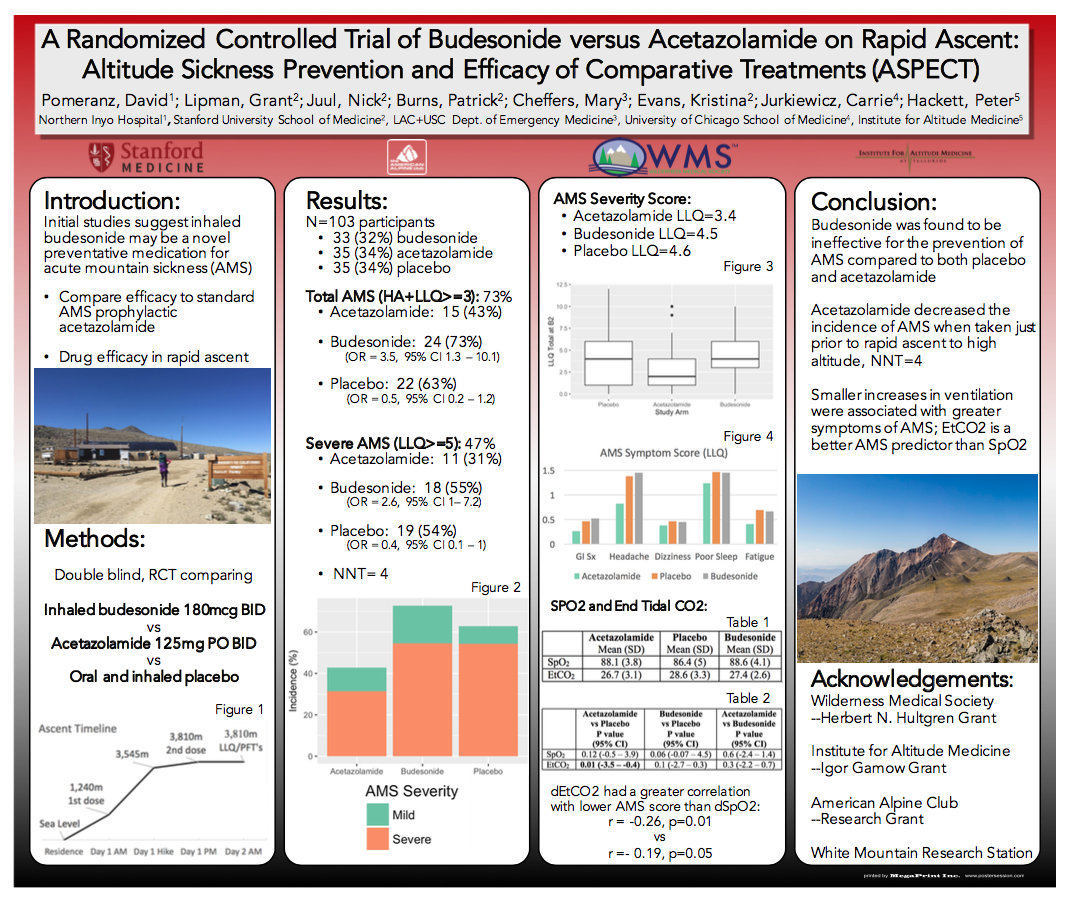
Background: Inhaled budesonide has been suggested as novel preventive medication for acute mountain sickness (AMS). However, efficacy has not been validated nor compared to the standard AMS prevention medication acetazolamide.
Methods: This double-blind, randomized, placebo-controlled trial compared inhaled budesonide to oral acetazolamide to placebo, starting the morning of ascent from 1,240 m (4,100 ft) to 3,810 m (12,570 ft) over 4 hours.
Results: 103 participants were enrolled and completed the study; 33 (32%) received budesonide, 35 (34%) acetazolamide, and 35 (34%) placebo. Demographics were not different between the groups (p > 0.09). Total AMS incidence was 73%, with severe AMS 47%. Fewer participants in the acetazolamide group (n=15, 43%) developed AMS compared to both budesonide (n=24, 73%) (OR = 3.5, 95% CI 1.3 – 10.1) and placebo (n=22, 63%) (OR = 0.5, 95% CI 0.2 – 1.2). Severe AMS was reduced with acetazolamide (n=11, 31%) compared with both budesonide (n=18, 55%) (OR = 2.6, 95% CI 1 – 7.2), and placebo (n=19, 54%) (OR = 0.4, 95% CI 0.1 – 1), with a number needed to treat of 4.
Conclusion: Budesonide was ineffective for the prevention of AMS, and acetazolamide was preventive of severe AMS taken just prior to rapid ascent.