Abstract
Introduction
Altered choline metabolism is a characteristic of prostate cancer and imaging using positron emission probes based on radiolabelled analogues (18F, 11C) of choline have shown promise in both the delineation of intra-prostatic and extra- prostatic extension of prostate cancer. Multi-parametric MRI imaging has demonstrated good to excellent performance in lesion detection. For these reasons the combination of 18-Fluorocholine (18FCH) PET and MRI imaging is attractive but has been hampered by technical challenges in fusion of these disparate imaging modalities. We describe our early experience imaging patients on a hybrid PET/MRI scanner with 18F-FCH and to our knowledge these are the first patients in North America so imaged.
Materials and methods
Patients were imaged as part of two prospective Health Canada approved clinical trials. In the first trial (IGPC-2) pre-operative multi-modality imaging is acquired for correlation with histopathology; in the second trial (IGPC-3) men with suspected recurrence post prostatectomy are imaged with 18F-FCH PET/MRI and the impact of the scan results on plan of management (compared to conventional imaging) is tracked.
Hybrid PET/MRI unit
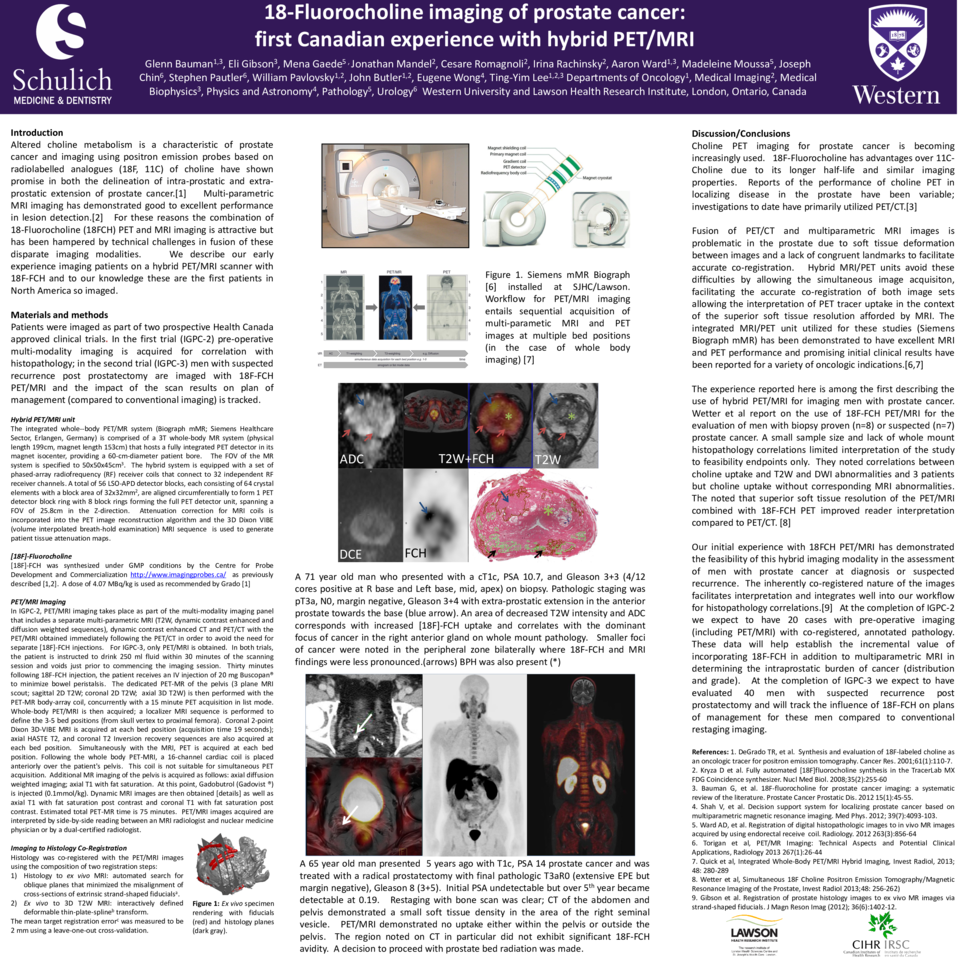
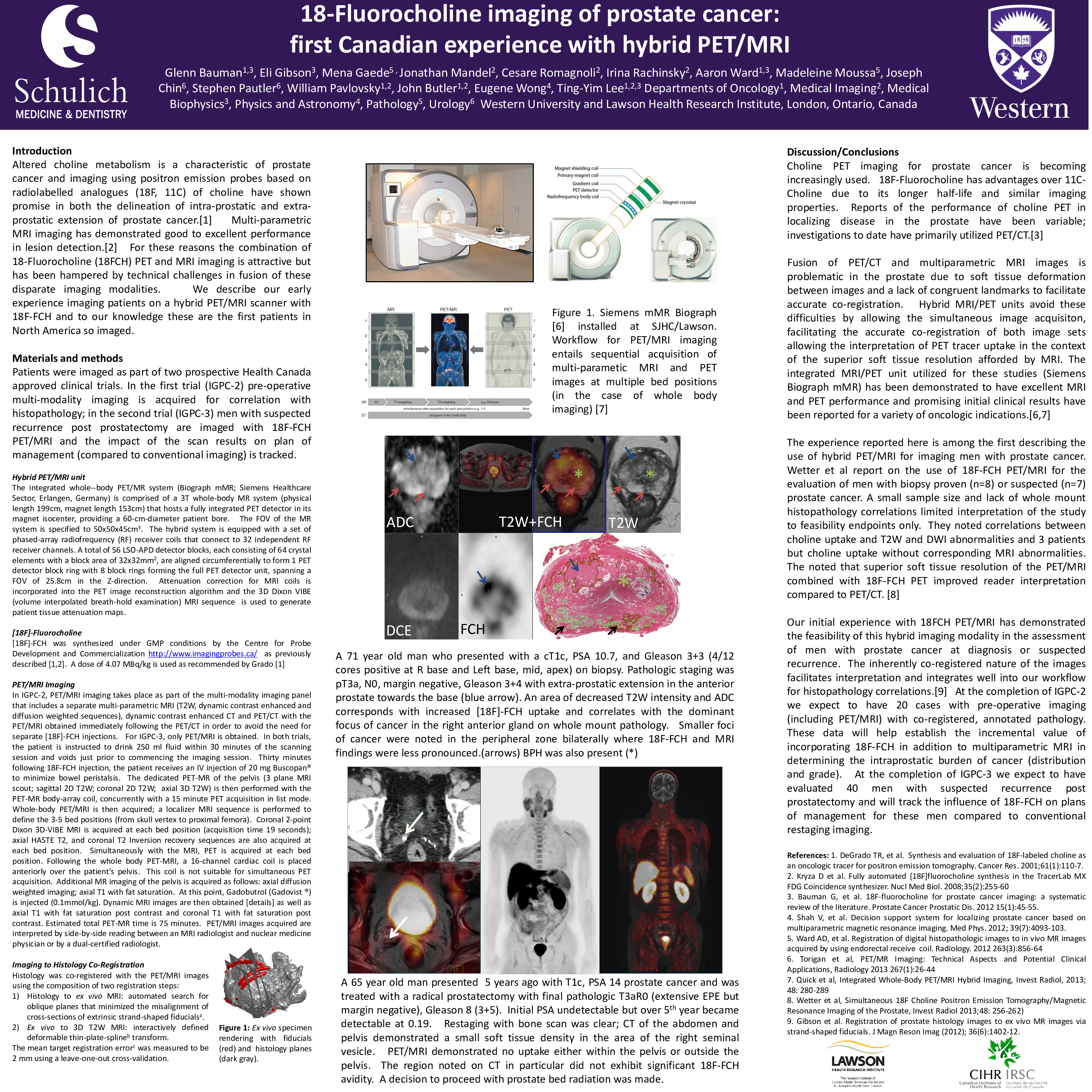
The integrated whole--body PET/MR system (Biograph mMR; Siemens Healthcare Sector, Erlangen, Germany) is comprised of a 3T whole-body MR system (physical length 199cm, magnet length 153cm) that hosts a fully integrated PET detector in its magnet isocenter, providing a 60-cm-diameter patient bore. The FOV of the MR system is specified to 50x50x45cm3. The hybrid system is equipped with a set of phased-array radiofrequency (RF) receiver coils that connect to 32 independent RF receiver channels. A total of 56 LSO-APD detector blocks, each consisting of 64 crystal elements with a block area of 32x32mm2, are aligned circumferentially to form 1 PET detector block ring with 8 block rings forming the full PET detector unit, spanning a FOV of 25.8cm in the Z-direction. Attenuation correction for MRI coils is incorporated into the PET image reconstruction algorithm and the 3D Dixon VIBE (volume interpolated breath-hold examination) MRI sequence is used to generate patient tissue attenuation maps.
[18F]-Fluorocholine
[18F]-FCH was synthesized under GMP conditions by the Centre for Probe Development and Commercialization http://www.imagingprobes.ca/ as previously described [1,2]. A dose of 4.07 MBq/kg is used as recommended by Grado [1]
PET/MRI Imaging
In IGPC-2, PET/MRI imaging takes place as part of the multi-modality imaging panel that includes a separate multi-parametric MRI (T2W, dynamic contrast enhanced and diffusion weighted sequences), dynamic contrast enhanced CT and PET/CT with the PET/MRI obtained immediately following the PET/CT in order to avoid the need for separate [18F]-FCH injections. For IGPC-3, only PET/MRI is obtained. In both trials, the patient is instructed to drink 250 ml fluid within 30 minutes of the scanning session and voids just prior to commencing the imaging session. Thirty minutes following 18F-FCH injection, the patient receives an IV injection of 20 mg Buscopan® to minimize bowel peristalsis. The dedicated PET-MR of the pelvis (3 plane MRI scout; sagittal 2D T2W; coronal 2D T2W; axial 3D T2W) is then performed with the PET-MR body-array coil, concurrently with a 15 minute PET acquisition in list mode. Whole-body PET/MRI is then acquired; a localizer MRI sequence is performed to define the 3-5 bed positions (from skull vertex to proximal femora). Coronal 2-point Dixon 3D-VIBE MRI is acquired at each bed position (acquisition time 19 seconds); axial HASTE T2, and coronal T2 Inversion recovery sequences are also acquired at each bed position. Simultaneously with the MRI, PET is acquired at each bed position. Following the whole body PET-MRI, a 16-channel cardiac coil is placed anteriorly over the patient's pelvis. This coil is not suitable for simultaneous PET acquisition. Additional MR imaging of the pelvis is acquired as follows: axial diffusion weighted imaging; axial T1 with fat saturation. At this point, Gadobutrol (Gadovist ®) is injected (0.1mmol/kg). Dynamic MRI images are then obtained [details] as well as axial T1 with fat saturation post contrast and coronal T1 with fat saturation post contrast. Estimated total PET-MR time is 75 minutes. PET/MRI images acquired are interpreted by side-by-side reading between an MRI radiologist and nuclear medicine physician or by a dual-certified radiologist.
Imaging to Histology Co-Registration
Histology was co-registered with the PET/MRI images using the composition of two registration steps: 1) Histology to ex vivo MRI: automated search for oblique planes that minimized the misalignment of cross-sections of extrinsic strand-shaped fiducials. 2) Ex vivo to 3D T2W MRI: interactively defined deformable thin-plate-spline transform. The mean target registration error was measured to be 2 mm using a leave-one-out cross-validation.
Discussion/Conclusions
Choline PET imaging for prostate cancer is becoming increasingly used. 18F-Fluorocholine has advantages over 11C- Choline due to its longer half-life and similar imaging properties. Reports of the performance of choline PET in localizing disease in the prostate have been variable; investigations to date have primarily utilized PET/CT.
Fusion of PET/CT and multiparametric MRI images is problematic in the prostate due to soft tissue deformation between images and a lack of congruent landmarks to facilitate accurate co-registration. Hybrid MRI/PET units avoid these difficulties by allowing the simultaneous image acquisiton, facilitating the accurate co-registration of both image sets allowing the interpretation of PET tracer uptake in the context of the superior soft tissue resolution afforded by MRI. The integrated MRI/PET unit utilized for these studies (Siemens Biograph mMR) has been demonstrated to have excellent MRI and PET performance and promising initial clinical results have been reported for a variety of oncologic indications.
The experience reported here is among the first describing the use of hybrid PET/MRI for imaging men with prostate cancer. Wetter et al report on the use of 18F-FCH PET/MRI for the evaluation of men with biopsy proven (n=8) or suspected (n=7) prostate cancer. A small sample size and lack of whole mount histopathology correlations limited interpretation of the study to feasibility endpoints only. They noted correlations between choline uptake and T2W and DWI abnormalities and 3 patients but choline uptake without corresponding MRI abnormalities. The noted that superior soft tissue resolution of the PET/MRI combined with 18F-FCH PET improved reader interpretation compared to PET/CT.
Our initial experience with 18FCH PET/MRI has demonstrated the feasibility of this hybrid imaging modality in the assessment of men with prostate cancer at diagnosis or suspected recurrence. The inherently co-registered nature of the images facilitates interpretation and integrates well into our workflow for histopathology correlations. At the completion of IGPC-2 we expect to have 20 cases with pre-operative imaging (including PET/MRI) with co-registered, annotated pathology. These data will help establish the incremental value of incorporating 18F-FCH in addition to multiparametric MRI in determining the intraprostatic burden of cancer (distribution and grade). At the completion of IGPC-3 we expect to have evaluated 40 men with suspected recurrence post prostatectomy and will track the influence of 18F-FCH on plans of management for these men compared to conventional restaging imaging.