Abstract
Purpose: Prostate Specific Membrane Antigen (PSMA) Positron Emission Tomography/Computed Tomography (PET/CT) can have positive findings for patients with prostate cancer, even when conventional imaging (CI) is negative.
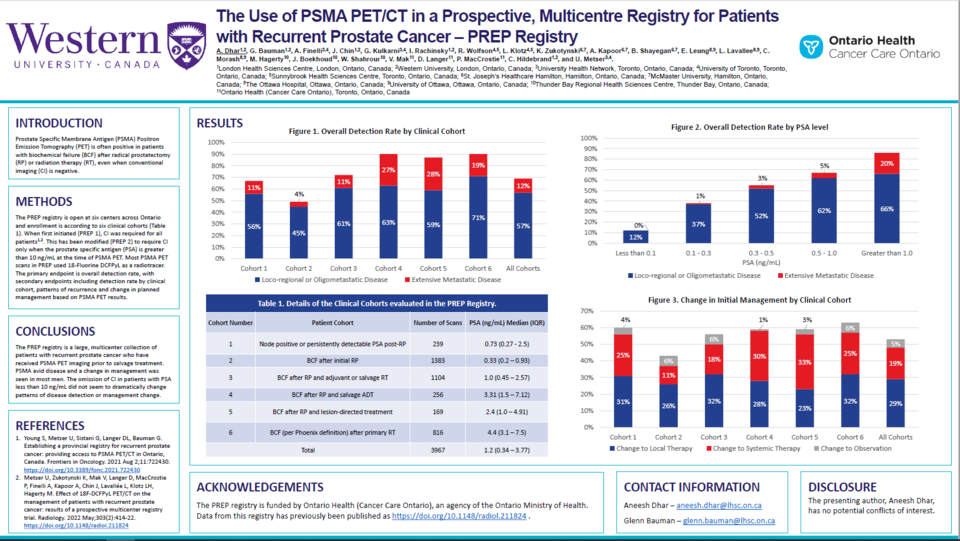
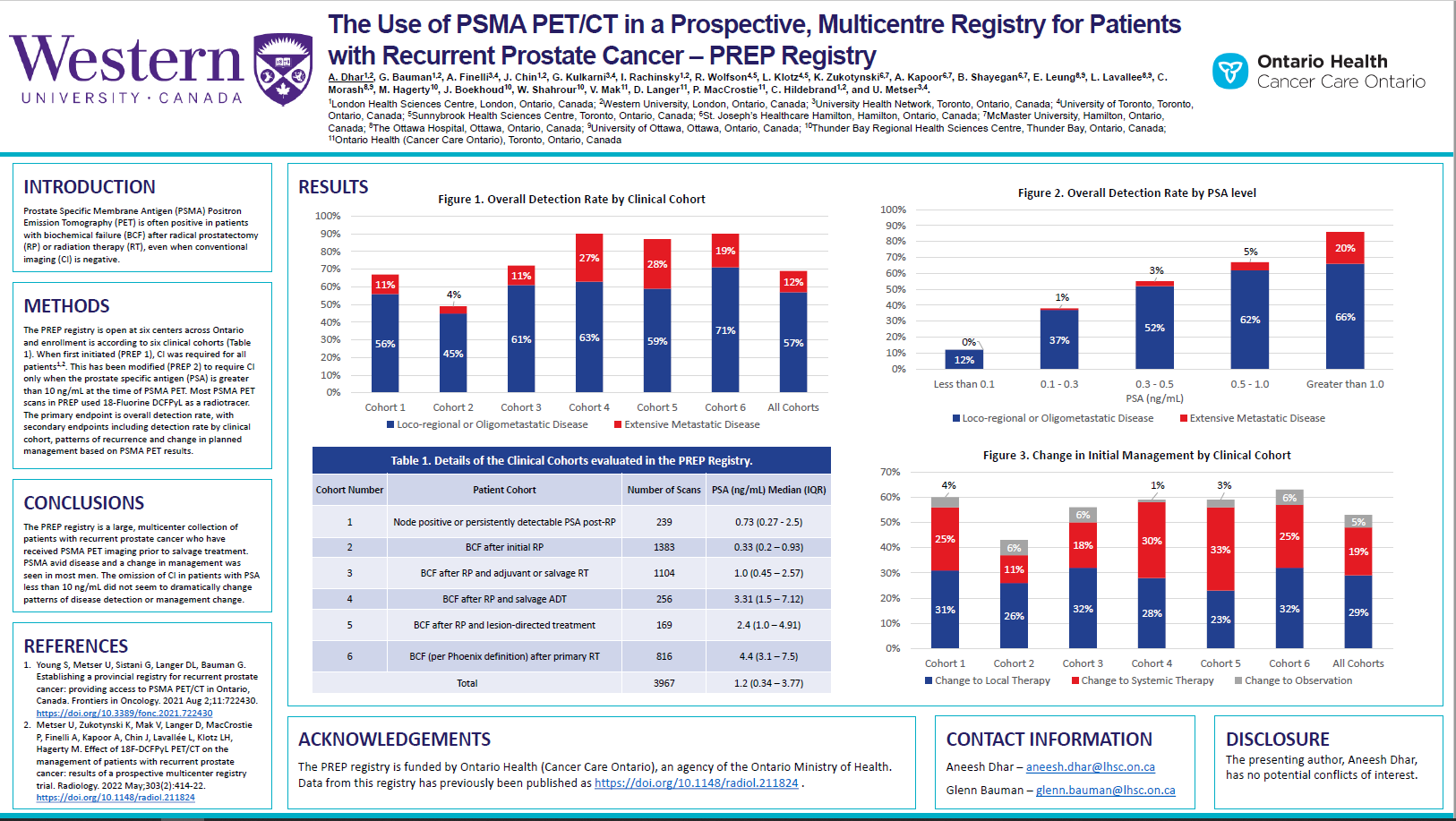
Materials and Methods: PREP is a prospective registry open at five Ontario centres. Enrollment is according to six clinical cohorts: 1) Node positive disease or persistently detectable prostate specific antigen (PSA) post-radical prostatectomy (RP); 2) biochemical failure (BCF) after initial RP; 3) BCF after initial RP and adjuvant or salvage radiation therapy (RT); 4) BCF after RP and salvage ADT; 5) BCF after prior PSMA PET lesion-directed therapy; and 6) BCF (per Phoenix definition) after definitive RT. All PSMA PET/CTs used 18F DCFPyL as a radiotracer. CI was required for all patients initially (PREP1), but this has been modified (PREP 2) to require CI only when PSA is greater than 10 ng/mL. The primary endpoint is overall detection rate, with secondary endpoints including detection rate by clinical cohort, patterns of recurrence and change in planned management based on PSMA PET/CT results.
Results: From December 2018 to March 2022, 3967 PSMA PET/CT studies were completed; 348 (12%) were repeat scans. Median age (Interquartile range (IQR)) for all cohorts was 71 (66–76) years. For cohorts 2, 3, and 6 (BCF after local therapy)[GB1] [AD2] , the median PSA (IQR) was 0.33 (0.2–0.93) ng/mL, 1.0 (0.45–2.57) ng/mL, and 4.4 (3.1–7.5) ng/mL. For these cohorts, the overall detection rate was 49, 72 and 90%, with limited (pelvic only or oligometastatic) disease detected in 45, 61, and 71% of scans and extensive metastatic disease detected in 4, 11, and 19% of scans. When grouped by initial PSA, the overall, limited disease and extensive disease detection rates in all six cohorts were the following: for PSA less than 0.1 ng/mL, 12, 12, and 0%; PSA between 0.1–0.3 ng/mL, 38, 37, and 1%; PSA between 0.3–0.5 ng/mL, 55, 52, and 3%; PSA between 0.5–1.0 ng/mL, 67, 62, and 5%; and for PSA greater than 1.0 ng/mL, 88, 68, and 20%. In all cohorts, when PSA was less than 10 ng/mL, the overall detection rates, with or without CI, were similar (64 vs 70%), as were changes in management (59 vs 50%). For cohorts 2, 3, and 6, the PSMA PET/CT changed management in more than half of cases: 26, 32, and 32% of cases changed management to local salvage; 11, 18, and 25% changed management to systemic therapy; and 6, 6, and 6% changed management to observation.
Conclusions: PREP is a multicentre registry of patients with recurrent prostate cancer who received PSMA PET/CT. The detection rate increased with increasing PSA levels; half of scans with PSA greater than 0.3 ng/mL had positive findings. The omission of CI in patients with PSA less than 10 ng/mL did not dramatically change patterns of disease detection or management change. A change in management were seen in most men after PSMA PET/CT.