Abstract
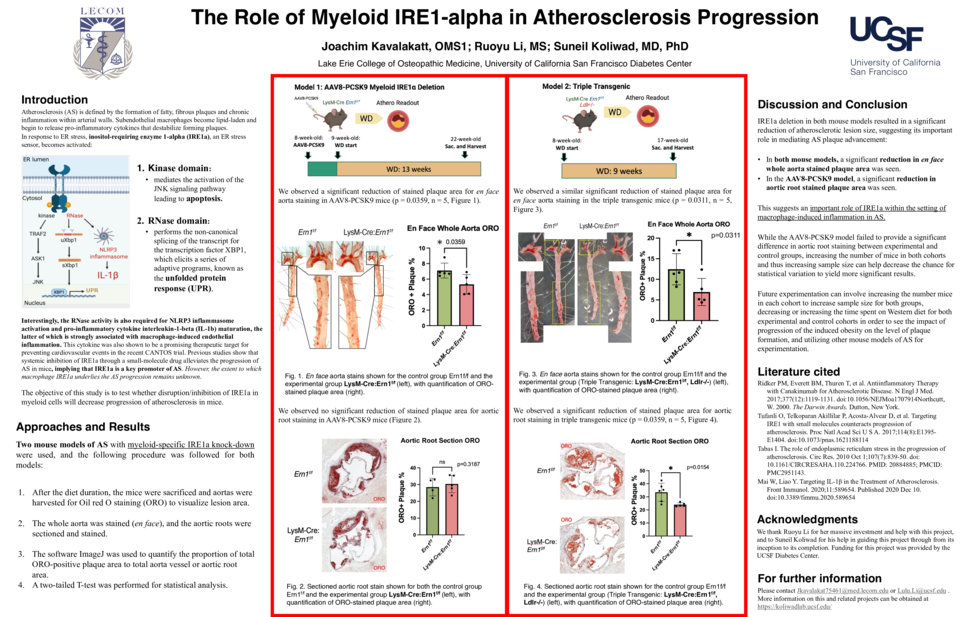
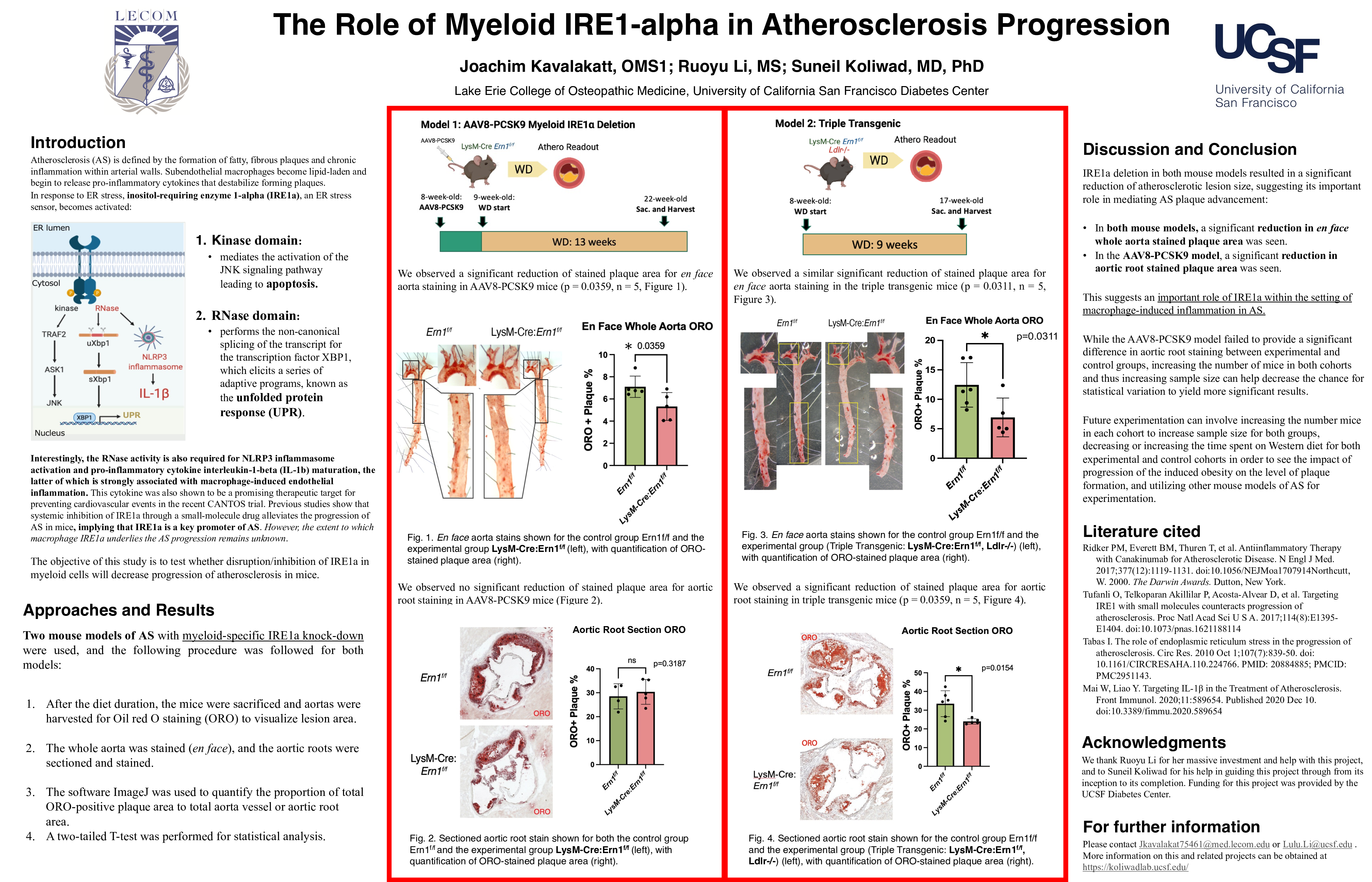
Background: Atherosclerosis (AS) is defined by the formation of fatty, fibrous plaques and chronic inflammation within arterial walls. In healthy vasculature, intimal macrophages maintain vascular homeostasis by engulfing lipids (e.g., oxidized LDLs). In a disease state, macrophages become lipid-laden and begin to release pro-inflammatory cytokines that destabilize forming plaques. In response to ER stress, inositol-requiring enzyme 1-alpha (IRE1a), an ER stress sensor, becomes activated. IRE1a’s kinase domain mediates the JNK signaling pathway leading to apoptosis. It also has an RNase domain that performs the non-canonical splicing of the transcript for the transcription factor XBP1, which subsequently elicits a series of adaptive programs, known as the unfolded protein response. Interestingly, the RNase activity is also required for NLRP3 inflammasome activation and pro-inflammatory cytokine interleukin-1-beta (IL-1b) maturation, the latter of which was shown to be a promising therapeutic target for preventing cardiovascular events in the recent CANTOS trial. A previous study showed that systemic inhibition of IRE1a through a small-molecule drug alleviates the progression of AS in mice, implying that IRE1a is a key promoter of AS. However, the extent to which macrophage IRE1a underlies the AS progression remains unknown.
Objective: The objective of this study is to test whether disruption/inhibition of IRE1a in myeloid cells will decrease progression of atherosclerosis in mice.
Methods: This study utilized two mouse models of AS with myeloid-specific IRE1a knock-down. Model 1– AAV8-PCSK9: The administration of AAV8(adeno-associated virus 8)-PCSK9 (proprotein convertase subtilisin/kexin type 9) construct promotes LDL receptor degradation. The control group Ern1f/f (n = 5) and the experimental group LysM-Cre: Ern1f/f (n=5) were both injected with the AAV and put on a Western diet for 13 weeks.
Model 2 – Triple Transgenic: This mouse model was developed by crossing LysM-Cre: Ern1f/f mice into a Ldlr-/- background. The control group Ern1f/f: Ldlr-/- (n = 6) and experimental group LysM-Cre: Ern1f/f: Ldlr-/- (n = 5) were put on a Western diet for 9 weeks.
After the diet duration, the mice were sacrificed, and aortas were harvested for Oil red O staining (ORO) to visualize lesion area. The whole aorta was stained (en face), and the aortic roots were sectioned and stained. The software ImageJ was used to quantify the proportion of total ORO-positive plaque area to total aorta vessel or aortic root area. A two-tailed T-test was performed for statistical analysis.
Results: We observed a significant reduction of stained plaque area for en face aorta staining in AAV8-PCSK9 mice (p = 0.0359, n = 5) and triple transgenic mice (p = 0.0311, n = 5) models. Aortic root staining in the triple transgenic model showed significant lesion reduction (p = 0.0154, n = 5).
Conclusion: IRE1a deletion in both mouse models resulted in a significant reduction of atherosclerotic lesion size, suggesting its important role in mediating AS plaque advancement.