Abstract
Purpose/Objectives
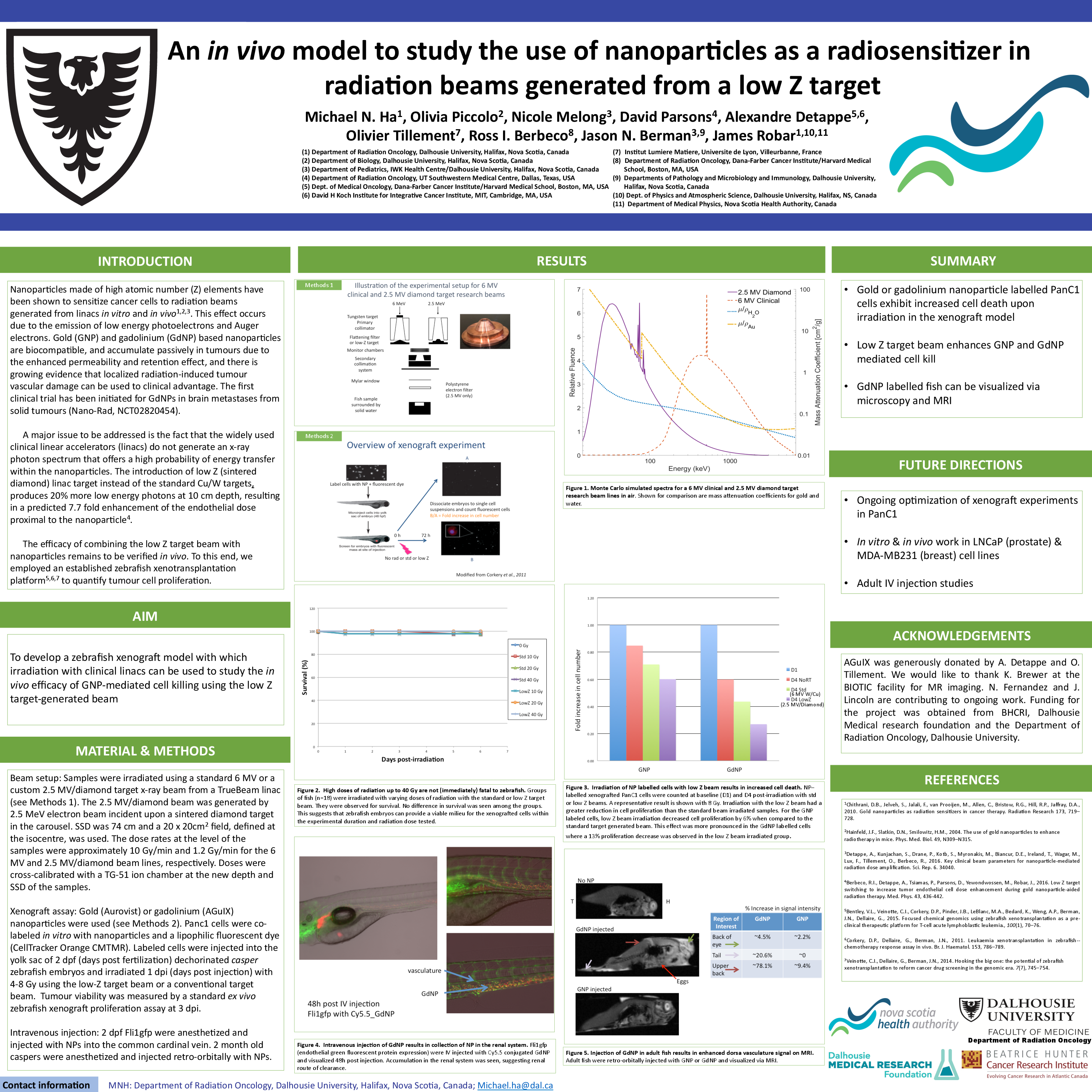
Nanoparticles made of high atomic number (Z) elements have been shown to sensitize cancer cells to radiation beams generated from linacs in vitro and in vivo. This effect occurs due to the emission of low energy photoelectrons and Auger electrons. Gold (GNP) and gadolinium (GdNP) based nanoparticles are biocompatible, and accumulate passively in tumours due to the enhanced permeability and retention effect, and there is growing evidence that localized radiation-induced tumour vascular damage can be used to clinical advantage. The first clinical trial has been initiated for GdNPs in brain metastases from solid tumours (Nano-Rad, NCT02820454). However, a major issue to be addressed is the fact that the widely used clinical linacs do not generate an x-ray photon spectrum that offers a high probability of energy transfer within the nanoparticles. The introduction of low Z (sintered diamond) linac target instead of the standard Cu/W targets, produces 20% more low energy photons at 10 cm depth, resulting in a predicted 7.7 fold enhancement of the endothelial dose proximal to the nanoparticle. The efficacy of combining the low Z target beam with nanoparticles remains to be verified in vivo. To this end, we employed an established zebrafish xenotransplantation platform to quantify tumour cell proliferation.
Materials/Methods
Beam setup: The samples were irradiated using a standard 6 MV or a custom 2.5 MV/diamond target x-ray beam from a TrueBeam linac. The 2.5 MV/diamond beam was generated by 2.5 MeV electron beam incident upon a sintered diamond target in the carousel. The dose rates at the level of the samples were approximately 10 Gy/min and 1.2 Gy/min for the 6 MV and 2.5 MV/diamond beam lines, respectively.
Xenograft assay: Gold (Aurovist) or gadolinium (AGuIX) nanoparticles were used. Panc1 cells were co-labeled in vitro with nanoparticles and a lipophilic fluorescent dye (CellTracker Orange CMTMR). Labeled cells were injected into the yolk sac of 2 dpf (days post fertilization) dechorinated casper zebrafish embryos and irradiated 1 dpi (days post injection) with 4-8 Gy using the low-Z target beam or a conventional target beam. Tumour viability was measured by a standard ex vivo zebrafish xenograft proliferation assay at 3 dpi.
Results
Up to 40 Gy of radiation was non-fatal to embryos during the timeline of the experiments (0-7 days). Xenograft experiments with GNP and GdNP labelled Panc1 cells revealed that irradiation with the low Z beam had a greater reduction in cell proliferation than the standard beam irradiated xenograft. For the GNP labeled cells, low Z beam irradiation decreased cell proliferation by 6% when compared to the standard target generated beam. This effect was more pronounced in the GdNP labelled cells where a 13% proliferation decrease was observed in the low Z beam irradiated group.
Conclusions
Gold or gadolinium nanoparticle labeled Panc1 cells exhibit increased cell death in vivo when exposed to low Z target-generated photon beam as predicted. Further experiments are planned in adult zebrafish.