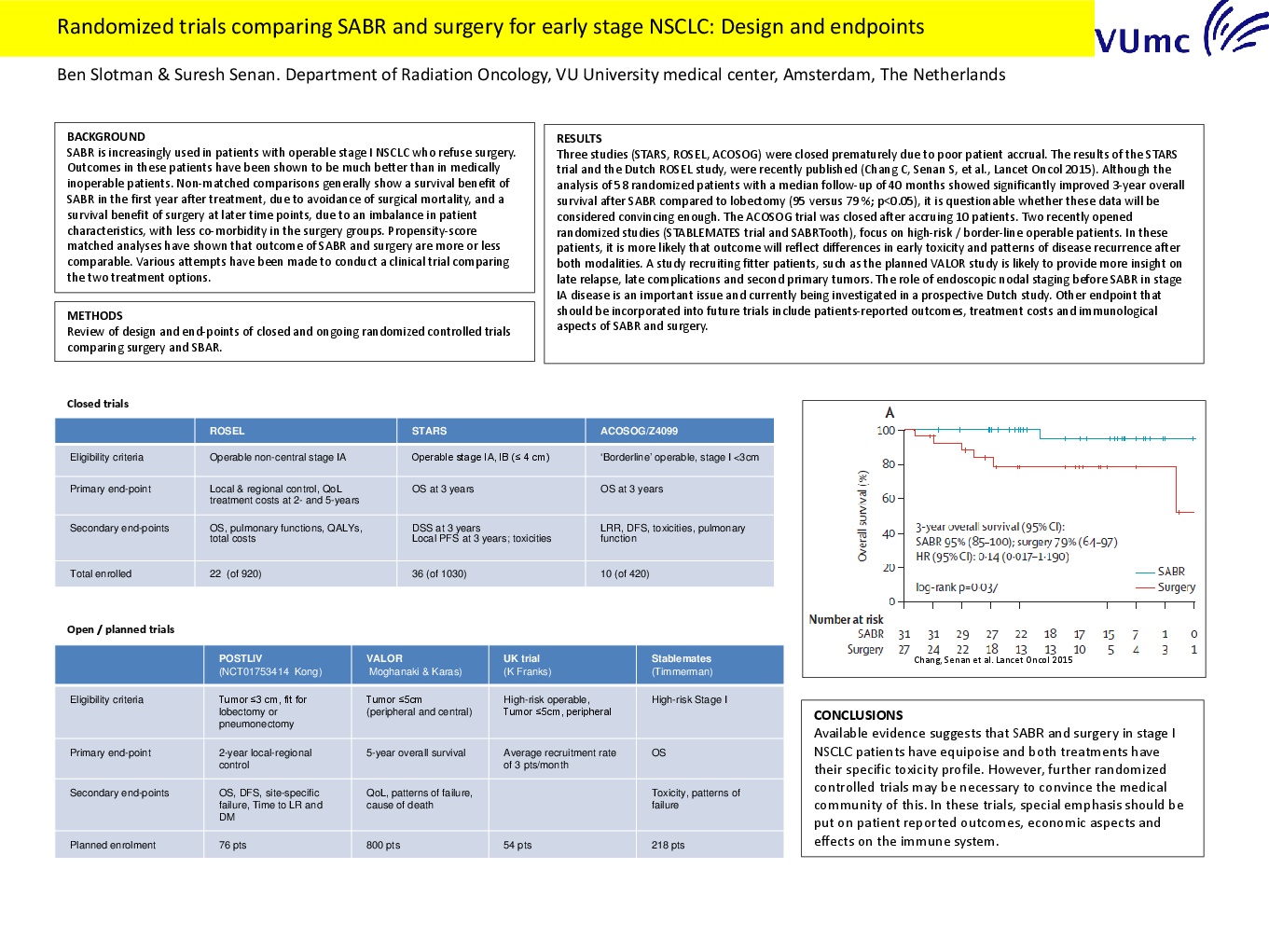
Abstract
Stereotactic ablative radiotherapy (SABR) is increasingly used in patients with operable stage I non-small cell lung cancer (NSCLC) who refuse surgery. Outcomes in these patients have been shown to be much better than in medically inoperable patients. Non-matched comparisons generally show a survival benefit of SABR in the first year after treatment, due to avoidance of surgical mortality, and a survival benefit of surgery at later time points, due to an imbalance in patient characteristics, with less co-morbidity in the surgery groups. Propensity-score matched analyses have shown that outcome of SABR and surgery are more or less comparable. Various attempts have been made to conduct a clinical trial comparing the two treatment options. In this report, we reviewed the design and end-points of closed and ongoing randomized controlled trials comparing surgery and SABR.